Choosing the Right Fluorescent Protein
In 1994 the green fluorescent protein cloned from Aequorea victoria became the first in a long line of genetically encoded labels. Since that time, the fluorescent protein palette has expanded to cover the entire visual spectrum. With so many color variations and options, which fluorescent protein (FP) is best for your research? Three key factors are among the most important to consider: brightness, photostability, and aggregation.
Brightness is the most obvious factor that most researchers consider when choosing an FP. In general, the brighter the FP, the better it will perform under almost all experimental conditions. When evaluating an FP’s brightness, make sure to look at the critical optical parameters — extinction coefficient and quantum yield. The product of these two values for different FPs can be used to directly compare their brightness. Brighter FPs will have lower detection limits (i.e. the concentration at which the FP becomes visible above autofluorescence of other cell components), and will allow imaging with lower excitation light intensity, minimizing the possibility of phototoxic effects.
Photostability has increasingly become a consideration when researchers choose fluorescent proteins. Many FPs, even if they are initially quite bright, will photobleach under continuous excitation during imaging. In order to perform long-term imaging experiments or to do quantitative analysis, an FP with high photostability should be the first choice. Unfortunately, methods for measuring and reporting photostability vary widely in the scientific literature, so be sure to understand how your FP’s photostability was measured before trying to make comparisons!
Aggregation (or oligomerization) has been one of the major issues tackled in the development of FPs. Many wild-type FPs form tetramers, which aggregate badly when expressed as fusion tags in cells. Engineered monomeric forms of many FPs are now available, and these monomeric FPs should always be used when making fusion constructs. For simple expression markers, however, oligomerization is not usually a major concern, and the brightest possible FP should be used in this case.
As with other research tools, doing your homework and reading the primary literature is always the best approach to choosing the right FP for your project!
Social Media for the Consumer
Business has become an ever changing, dynamic landscape where the power and information no longer lies strictly in the hands of “the right people.” In less than a decade Facebook has managed to link nearly a billion people from tech savvy teens to curious senior citizens. Similarly, Twitter sees over 340 million tweets that reaches over 500 million active users per day. Social media is a wide reaching technology with infinite possibilities that has been synthesized and is fueled by people, not corporations.
In a recent Industry report, 93% of businesses reported using social media as a marketing tool with the benefits being increased exposure and customer interaction (Stelzner, 2011). What does this study mean to consumers? Businesses are coming to you and responding to your feedback. Social media gives you, the consumer; back the power and the opportunity to benefit from your loyalty. Most businesses, from restaurants to labs, offer weekly tips or discounts on their services to the customers who “follow” or “like” them. Here at Allele Biotech, we have begun offering special weekly promotions, information about our products and interesting biotech news to our customers who either “follow” us on Twitter or “like” us on Facebook. For the entire month of August we are offering 15% off just for a “like” or “follow.” If you are one of the billion users, it doesn’t take much more than a click for instant rewards.
We recognize that the reason we create this technology and improve is because of the consumers. Allele Biotech would like to thank you for your loyalty and encourage you to remain active within our social media to push us forward and to help us continuously strive for more. With the creation and exponential popularity of social media, the power and information as become available to the most important people, the consumers.
Twitter @Allele_Biotech
Facebook http://www.facebook.com/pages/Allele-Biotechnology-and-Pharmaceuticals-Inc/78331924957
Cord Banking and iPS Cells
Umbilical Cord Banking (UCB) has been a popular discussion topic in the United States since the first Cord Bank was established in New York in 1992. Since the first cord blood transplantation in 1988, there have been over 780,000 UCB donations to private banks and 400,000 UCB donations to public blood banks worldwide. There has been such a great number of donations because UCB is full of hematopoietic progenitor cells, which makes it a more desirable solution to genetic, metabolic and immune disorders, over bone marrow and blood. Because of the nature of UCB, the recipient does not need to be an immunological match, there is a lower rate of infection and it is much easier to acquire than bone marrow, making it the ideal form of treatment for many patients and practitioners.
Over twenty years later, a new technology is emerging that could provide some clarity to the “to donate or not to donate” debate: induced pluripotent stem cells (iPSC). Derived from adult cells, iPSCs have the potential to be used like UCB or reprogrammed into specific tissue like myocytes. This potential opens up banking to countless individuals born before 1992, who never had an option to bank their UCB. With this unbounded potential, should iPSCs be banked liked UCB? Supporters argue that there has been enough evidence thus far to start a bank, however, most people seem to agree that too much is unknown about iPSCs and their use in humans. With that, most are in agreement that iPSC research is absolutely needed so banking can become a reality in the future.
For now iPSCs will remain in the testing and research phase, however, based on current research, iPSCs have the potential to enhance Cord Blood that has already been banked, perhaps providing some relief to public banks in the future (Broxmeyer, 2010). Though the potential of iPSCs is endless, more work has to be done before they are placed in humans and considered a viable banking system.
VHH Nanobodies in Superresolution Imaging and More
From the large number of recent publications using GFP-Trap beads, it appears that GFP-Trap is on the way to becoming one the most popular tags for co-IP thanks to its unparalleled “cleanness” of precipitated protein bands and its quantitative binding capabilities. As described previously, the antibody conjugated on the GFP-Trap beads is a single-domain antigen binding module from camelid single-chain antibodies. Termed VHH, this domain is only ~12 kD and can fit into structures that other types of antibodies cannot. We have successfully created VHH antibodies against a number of neural factors as a research project for the NIDA/NIH.
VHH antibodies are often called nanobodies as a result of their size (1.5 – 2.5nm) and binding affinity ( GFP-trap has a binding affinity of 0.59nM). In addition to their use for co-IP, VHH antibodies have proven themselves as a resilient tool for various other applications. Anti-GFP nanobodies, for example, are currently used to enhance the fluorescence of GFP (GFP-trap booster utilizes the same VHH binding antibody coupled to a fluorescent dye); others have used VHH antibodies that can insert into certain part of GFP to dim the fluorescence signal . More recently, Ries et al. published in Nature Methods that the anti-GFP nanobodies offered a simple and versatile method for super-resolution imaging (i.e. PALM)-previously super-resolution imaging requires photoconvertible fluorescent proteins (such as Eos, mClavGR2). With dye-conjugated nanobodies, generating fusions to these newer FPs is no longer needed, however, using the nanobody super-imaging method requires fixing and permeabilizing the cells.
When using anti-GFP VHH reagents you need to be aware that other fluorescent proteins can also be recognized, if they were derived from the avGFP (jellyfish GFP). Also, some GFPs are not recognized if they are from another species, or engineered such as our mWasabi. We are producing newer and brighter GFP/YFPs based on the lancelet YFP protein to offer alternative series that will not be cross-recognized by the GFP-Trap antibodies.
Picture Blog — Making mRNAs by In Vitro Transcription for Transgene Expression and R-iPSCs
R-iPS Cell FAQ 2:
What is the expected yield from the in vitro trancription (IVT) reactions?
Performed as described, you should recover around 40 ug RNA from each 40 uL IVT reaction.
R-iPS Cell FAQ 3:
How can the success of the RNA synthesis protocol be assessed?
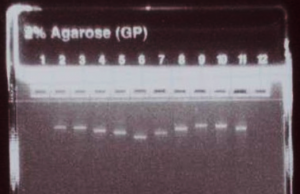
Run 500 ng (5 uL) of the concentration-adjusted products on an E-gel to check for consistent product yield and relative product sizes, and to confirm the absence of secondary bands or smears.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008



