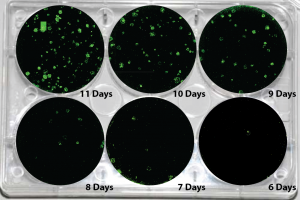
Picture Blog — Human mRNA-Induced Pluripotent Stem Cells Generated in Days
R-iPS Cell FAQ 1:
What phenotypic changes can be observed during a successful reprogramming trial?
About one week out, target fibroblasts should show an involution of fibroblastic processes, and foci or clusters of epitheliod cells—ideally with small nuclei, minimal cytoplasm, and signs of ongoing mitosis—should appear. Colonies with hESC morphology typically start emerging in ~10-14 days.
Using Insect Cells For Making Mammalian Proteins
Recombinant protein expression is a major part of biological research. In theory, once the genetic code of a protein is known from cDNA analysis or whole genome sequencing, any polypeptide of interest, existing in nature or perceived, can be artificially produced. Bacteria cells are commonly used to express a variety of proteins because they are more convenient and less costly than other systems. However, a significant percentage of proteins naturally expressed in mammalian cells are not soluble or cannot be easily produced in bacteria such as E. coli. Like bacteria, yeasts are also easy to culture and manipulate, however, although they are eukaryotes, they are not capable of adding “mammalian-like” post-translation modifications (PTM). Insect cells can be used effectively for producing large quantities of mammalian proteins rather easily through baculovirus such as Allele´s Sapphire system. PTM in insect cells is not exactly the same as in mammalian cells, e.g. different glycosylation patterns, but is a lot closer than yeasts. Mammalian cells are used for proteins that require appropriate PTM or are not soluble in other systems through either transient transfection or stable cell line establishment.
For protein expression in insect cells, a number of factors need to be taken into consideration:
1) Genomic DNA for creating baculovirus stocks that will ensure a high percentage of recombinant virus (to avoid wild-type, non-producing virus)
2) Transfer plasmid for cloning the protein-encoding cDNA for easy cloning and appropriate co-expression of helper or marker proteins (such as through insect IRES)
3) Cell lines that have the highest expression levels of a particular protein, sometimes a number of cell lines need to be screened
4) Cell medium, because insect cell medium may contain high levels of ions that can interfere with affinity tag-based purification, one needs to find the most appropriate medium for protein expression
5) Secreted vs nonsecreted proteins. Insect cells need to have their own secretion signal (and translation signal, IRES, polyadinylation, etc.)
More reading…http://www.allelebiotech.com/protein-expression-in-insect-cells/
Opportunities for business with Allele Biotech
Allele Biotech is known for staying on the edge of biological research fronts when it comes to developing new technologies into useful tools. Our research also has far-reaching implications and potential applications outside of the traditional biomedical research reagent field. Some of these technologies were the results of researchers interacting with the Allele scientific team, who wanted Allele to help realize their potentials. If you are interested in investing, co-developing, or trading in our areas of expertise, please email us at oligo@allelebiotech.com.
1) A novel method of discriminating and/or detecting mismatched polynucleotide populations in a sample, or determining the relative abundance of the species contained in the sample based on the changes in the relative ratios following a critical treatment. This technology, subject of a current patent application, can provide great benefits in polynucleotide-based diagnosis.
2) A technology on how to utilize the light-absorbing capabilities of certain light-absorbing proteins against damaging lights, or in cosmetic or beauty products. It is also a subject of a filed full patent.
3) Products that relate to detecting swine flu with novel antibodies of high specificity and stability. The antibodies have been tested in academic molecular biology labs in ELISA and strip formats.
4) Nanotechnology products that can be immediately applied to prevent citrus diseases on farms.
5) Enzymes as additives to animal feeds that help farm animals digest. The product is already being sold in certain regions.
Making Transfection-Grade mRNA by IVT (In Vitro Transcription)
RNases are an often feared in molecular biology labs because of their high stability and ominous presence in virtually all living systems. Consequently, people who work with RNA are trained to exercise extreme caution to avoid RNA degradation: change gloves often because human hands ooze RNases; use only sterilized labware as microbes may be sources of RNases; for surfaces that can’t be autoclaved, use sprays like “RNase Zap” (SDS- or guanidine-containing solutions). Such cautionary steps are especially necessary when dealing with low abundance RNA samples.
RNAs can be produced by in vitro transcription (IVT), a simple reaction requiring only a DNA template (double-stranded or even single-stranded DNA as long as the promoter region is double-stranded), RNA polymerase (from T7, SP6, or T3 phage), NTPs, and a reaction buffer that provides appropriate salt and pH. Standard NTPs may be replaced with modified ones to either increase stability or to reduce immune-response when transfected into cultured cells. Additionally, a 5’ cap structure may be added during IVT for further stabilizing mRNAs inside the cells post transfection. Using a commercially assembled kit, one can routinely produce 40-50 µg of mRNA from 1 µg of DNA template in a single 20-50 µl reaction.
At such high concentrations, IVT mRNAs are not nearly as sensitive to RNase-mediated degradation as low-abundance samples. The mRNA can be easily observed on agarose gels that are regularly used for DNA, and their integrity can be monitored after transcription or storage. In most cases one distinct band of mRNA from an IVT reaction is obtained as long as a clean DNA template is used. Preparing a good, uniform IVT template is critical to prevent aberrant products. By using high quality templates, IVT mRNA produced in your own lab are often higher in quality than mRNAs purchased from current commercial sources (Figure in Blog shows mRNAs generated by IVT for R-iPSC). Sometimes there are minor bands created during IVT, but they normally do not interfere with the intended uses of the mRNA, and can be purified away with a purification kit (by using a discriminating purification scheme such as Allele Biotech’s Surface Bind RNA Purification, smaller species can be specifically removed, a separate topic for another blog).
Once produced, mRNAs can be stored at -20C for months, or -80C nearly indefinitely.
Record number of papers citing the GFP-Trap group products in 2011
The following are references in regards to GFP Trap published in the second half of 2011 (not a complete list); a high quality GFP-binding protein based on a single domain antibody derived from Camelids. It is characterized by a small barrel shaped structure (13 KDa, 2.5nm X 4.5 nm) and a very high stability (stable up to 70°C, functional within 2M NaCl or 0.5% SDS). With much greater stability, specificity, and affnity, GFP-Trap®, the recent addition to antibodies for immunoprecipitation, should make GFP the most suitable tag for immunoprecipitation assays.
For live PubMed links, view this version please.
Krastev, D. B., Slabicki, M., et al. (2011). A systematic RNAi synthetic interaction screen reveals a link between p53 and snoRNP assembly. Nature Cell Biology. 13: 809-818. PubMed
Aboobakar, E. F., Wang, X., et al. (2011). The C2 domain protein Cts1 functions in the calcineurin signaling circuit during high temperature stress responses in Cryptococcus neoformans. Eukaryotic Cell. EC. 05148-05111v05141. PubMed
Uhrig, R. G. and Moorhead, G. B. G. (2011). Two ancient bacterial-like PPP family phosphatases from Arabidopsis thaliana are highly conserved plant proteins that possess unique properties. Plant Physiology. PubMed
Larance, M., Kirkwood, K. J., et al. (2011). Characterization of MRFAP1 Turnover and Interactions Downstream of the NEDD8 Pathway. Molecular & Cellular Proteomics. PubMed
Hattersley, N., Shen, L., et al. (2011). The SUMO protease SENP6 is a direct regulator of PML nuclear bodies. Molecular Biology of the Cell. 22: 78-90. PubMed
Rancz, E. A., Franks, K. M., et al. (2011). Transfection via whole-cell recording in vivo: bridging single-cell physiology, genetics and connectomics. Nature Neuroscience. 14: 527-532. PubMed
Palmer, C. S., Osellame, L. D., et al. (2011). MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO reports. 12: 565-573. PubMed
Pichler, G., Wolf, P., et al. (2011). Cooperative DNA and histone binding by Uhrf2 links the two major repressive epigenetic pathways. Journal of Cellular Biochemistry. 112: 2585-2593. PubMed
Mitchell, L., Lau, A., et al. (2011). Regulation of Septin Dynamics by the Saccharomyces cerevisiae Lysine Acetyltransferase NuA4. PLoS One. 6: e25336. PubMed
Engeland, C. E., Oberwinkler, H., et al. (2011). The cellular protein Lyric interacts with HIV-1 Gag. Journal of virology. JVI. 00174-00111v00171. PubMed
Wang, C. and Youle, R. (2011). Predominant requirement of Bax for apoptosis in HCT116 cells is determined by Mcl-1’s inhibitory effect on Bak. Oncogene. PubMed
Tulloch, L. B., Howie, J., et al. (2011). The inhibitory effect of phospholemman on the sodium pump requires its palmitoylation. Journal of Biological Chemistry. 286: 36020-36031. PubMed
Sun, L. and Wang, C. C. (2011). The Structural Basis of Localizing Polo-Like Kinase to the Flagellum Attachment Zone in Trypanosoma brucei. PLoS One. 6: e27303. PubMed
Bouttier, M., Saumet, A., et al. (2011). Retroviral GAG proteins recruit AGO2 on viral RNAs without affecting RNA accumulation and translation. Nucleic acids research. PubMed
Matos, J., Blanco, M. G., et al. (2011). Regulatory Control of the Resolution of DNA Recombination Intermediates during Meiosis and Mitosis. Cell. 147: 158-172. PubMed
Nagel, C. H., Albrecht, N., et al. (2011). Herpes Simplex Virus Immediate-Early Protein ICP0 Is Targeted by SIAH-1 for Proteasomal Degradation. Journal of virology. 85: 7644. PubMed
Studencka, M., Konzer, A., et al. (2011). Novel roles of C. elegans heterochromatin protein HP1 and linker histone in the regulation of innate immune gene expression. Molecular and Cellular Biology.PubMed
Muehlen, S., Ruchaud-Sparagano, M. H., et al. (2011). Proteasome-independent Degradation of Canonical NFŒ?B Complex Components by the NleC Protein of Pathogenic Escherichia coli. Journal of Biological Chemistry. 286: 5100. PubMed
Galan, J. A., Paris, L. L., et al. (2011). Proteomic Studies of Syk-Interacting Proteins Using a Novel Amine-Specific Isotope Tag and GFP Nanotrap. Journal of the American Society for Mass Spectrometry. 1-10. PubMed
Chamousset, D., De Wever, V., et al. (2010). RRP1B Targets PP1 to Mammalian Cell Nucleoli and is Associated with Pre-60S Ribosomal Subunits. Mol Biol Cell. PubMed
Kovacs, E. M., Verma, S., et al. (2011). N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nature Cell Biology. 13: 934-943. PubMed
Boysen, K. E. and Matuschewski, K. (2011). Arrested oocyst maturation in Plasmodium parasites lacking type II NADH: ubiquinone dehydrogenase. Journal of Biological Chemistry. 286: 32661-32671. PubMed
Mortusewicz, O., Fouquerel, E., et al. (2011). PARG is recruited to DNA damage sites through poly (ADP-ribose)-and PCNA-dependent mechanisms. Nucleic acids research. 39: 5045. PubMed
Graewe, S., Rankin, K. E., et al. (2011). Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathogens. 7: e1002224. PubMed
Yang, X. D., Huang, S., et al. (2011). Distinct and mutually inhibitory binding by two divergent Œ?-catenins coordinates TCF levels and activity in C. elegans. Development. 138: 4255-4265. PubMed
Pollithy, A., Romer, T., et al. (2011). Magnetosome expression of functional camelid antibody fragments (nanobodies) in Magnetospirillum gryphiswaldense. Applied and environmental microbiology. 77: 6165-6171. PubMed
Kozubowski, L., Thompson, J. W., et al. (2011). Association of Calcineurin with the COPI Protein Sec28 and the COPII Protein Sec13 Revealed by Quantitative Proteomics. PLoS One. 6: e25280. PubMed
Garcia-Gomez, J. J., Lebaron, S., et al. (2011). Dynamics of the putative RNA helicase Spb4 during ribosome assembly in Saccharomyces cerevisiae. Molecular and Cellular Biology. 31: 4156-4164. PubMed
Van Damme, D., Gadeyne, A., et al. (2011). Adaptin-like protein TPLATE and clathrin recruitment during plant somatic cytokinesis occurs via two distinct pathways. Proceedings of the National Academy of Sciences. 108: 615. PubMed
Qvist, P., Huertas, P., et al. (2011). CtIP Mutations Cause Seckel and Jawad Syndromes. PLoS Genetics. 7: e1002310. PubMed
Labella, S., Woglar, A., et al. (2011). Polo Kinases Establish Links between Meiotic Chromosomes and Cytoskeletal Forces Essential for Homolog Pairing. Developmental Cell. PubMed
Harterink, M., Port, F., et al. (2011). A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nature Cell Biology. 13: 914-923. PubMed
Konopacki, F. A., Jaafari, N., et al. (2011). Agonist-induced PKC phosphorylation regulates GluK2 SUMOylation and kainate receptor endocytosis. Proceedings of the National Academy of Sciences.PubMed
Chuhma, N., Tanaka, K. F., et al. (2011). Functional connectome of the striatal medium spiny neuron. The Journal of Neuroscience. 31: 1183-1192. PubMed
Jackson, B. R., Boyne, J. R., et al. (2011). An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export. PLoS Pathogens. 7: e1002138. PubMed
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008