iPSCs and other stem cells
The 2012 Nobel Prize for Physiology or Medicine is Awarded to Cell Reprogramming Scientists
Monday Sir John B. Gurdon and Shinya Yamanaka shared this year’s Nobel Prize for physiology or medicine for work that revolutionized the understanding of how cells and organisms develop.
“By reprogramming human cells, scientists have created new opportunities to study diseases and develop methods for diagnosis and therapy.”
This is the 3rd time that a Nobel Prize is awarded on a technology that we chose as our area of research and made contributions to the field. The other two are RNA interference (2005) and fluorescent proteins (2008).
Path to Better Drugs through Disease-Specific iPSCs
Induced human pluripotent stem cells
The recent finding that pluripotency, the ability to differentiate into all cell types typically associated with embryonic stem cells, can be induced in somatic cells may be the molecular equivalent of the discovery of antibiotics or vaccines in the last century [1].
iPSC-based disease modeling
Recent studies have described the generation of induced pluripotent stem cells (iPSCs) from patients with a full range of genetically inherited or sporadic diseases, and in vitro differentiation of these iPSCs to cell types relevant to the disorder with certain disease features.
Example 1 (out of ~20): Progressive motor neuron loss during differentiation of iPSCs derived from spinal muscular atrophy (SMA) patients, reflecting developmental loss seen in the disease.
Example 2: iPSCs made from RETT syndrome give rise to glutamatergic neurons with fewer synapses than controls, a better treatment was found from a panel of candidates based on this model.
Example 3: Neurons differentiated from iPSCs that have been derived from early or late onset Alzheimer’s disease were shown to display different properties and potential interference points.
The identification of novel pathways or drugs that could prevent disease is the ultimate goal of the iPSC-based disease modeling approach.
Major steps towards efficient iPSC disease modeling
The first hurdle for feasible application of patient-specific disease modeling is to achieve efficient generation of iPSCs from large cohorts of patients quickly and at a low cost while eliminating “clonal variations”. As described in a recent publication [2], the Allele Biotechnology team has shown that human fibroblasts can be converted to stem cells in just over a week, achieving bulk conversion efficiency without any chromosome modifications. The process is also xeno-free and feeder-free, enabling both fundamental scientific research and clinical applications.
The next major advancements required for disease modeling are robust lineage-specific differentiation protocols that provide a large number of desired cells for drug testing and screening. Cardiomyocytes derived from iPSCs have been the best known example of large expansion; other cell types will become available in the near future. Allele Biotechnology has commenced differentiating iPSCs along several lineages using our own iPSCs of superior quality.
With cells of disease-matching tissue types derived from patients’ iPSCs, cell-based assays can be designed and developed using various assay formats. Allele Biotech’s leading capacities in fluorescence and bioluminescence, gene silencing, delivery vehicles and single-domain targeting agents will be of unmatched value to drug discovery partners.
1. Review: Wu, SM and Hochedlinger, K. “Harnessing the potential of induced pluripotent stem cells for regenerative medicine ” 2011, Nature Cell Biology, V13-5, 497-505.
2. Allele Biotech publication: Warren, L., Ni, Y., Wang, J. and Guo, X. “Feeder-Free Derivation of Human Induced Pluripotent Stem Cells with Messenger RNA” 2012, Nature’s Scientific Reports, doi:10.1038/srep00657.
For business development contact:
iPS@allelebiotech.com
858-587-6645
Fax 858-587-6692
www.allelebiotech.com
6404 Nancy Ridge Drive
San Diego, CA 92121
Related products for academic customers: Non-Integrating iPSC Generation Product Line http://www.allelebiotech.com/non-integrating-ipsc-generation/
New Product of the week: 6F mRNA Reprogramming Premix: $995 for 10 reprogramming!
Allele Biotechnology Announces New advance in production of human stem cells
This week in the journal Scientific Reports (Nature Publishing Group) scientists from Allele Biotechnology describe an important advance in the generation of stem cells capable of producing all the different tissues of the human body. In an article entitled “Feeder-Free Derivation of Human Induced Pluripotent Stem Cells with Messenger RNA,” Allele’s scientists present the fastest and safest method yet for converting ordinary human skin cells into “induced pluripotent stem cells” (iPSCs).
The scientific efforts were led by Dr. Luigi Warren, whose pioneering work on “footprint-free” reprogramming using messenger RNA was the foundation for Allele’s breakthrough. Through the united efforts of Dr. Warren and the scientists at Allele Biotechnology, his technique was re-engineered to increase cell conversion efficiency and eliminate any use of potentially unsafe reagents, while substantially reducing the time and effort needed to make stem cells. Dr. Warren believes that because of its advantages this technology “should become the method of choice for iPSC cell banking.”
According to Dr. Jiwu Wang, corresponding author on the paper and CEO of Allele Biotechnology, “This advance in stem cell derivation will enable both fundamental scientific research and clinical applications which has been the mission of Allele Biotechnology from its inception.”
Allele Biotechnology and Pharmaceuticals Inc. is a San Diego-based biotechnology company that was established in 1999 by Dr. Jiwu Wang and colleagues. A research based company specializing in the fields of RNAi, stem cells, viral expression, camelid antibodies and fluorescent proteins; Allele Biotechnology has always striven to offer products and services at the cutting edge of research.
Allele Biotechnology and Pharmaceuticals Inc.
Jiwu Wang, Ph.D., 858-587-6645 Ext 3
President and CEO
iPS@allelebiotech.com
fax: 858-587-6692
www.allelebiotech.com
Press release by BusinessWire. Also see Yahoo!News, Reuters, The Herald, etc.
Cord Banking and iPS Cells
Umbilical Cord Banking (UCB) has been a popular discussion topic in the United States since the first Cord Bank was established in New York in 1992. Since the first cord blood transplantation in 1988, there have been over 780,000 UCB donations to private banks and 400,000 UCB donations to public blood banks worldwide. There has been such a great number of donations because UCB is full of hematopoietic progenitor cells, which makes it a more desirable solution to genetic, metabolic and immune disorders, over bone marrow and blood. Because of the nature of UCB, the recipient does not need to be an immunological match, there is a lower rate of infection and it is much easier to acquire than bone marrow, making it the ideal form of treatment for many patients and practitioners.
Over twenty years later, a new technology is emerging that could provide some clarity to the “to donate or not to donate” debate: induced pluripotent stem cells (iPSC). Derived from adult cells, iPSCs have the potential to be used like UCB or reprogrammed into specific tissue like myocytes. This potential opens up banking to countless individuals born before 1992, who never had an option to bank their UCB. With this unbounded potential, should iPSCs be banked liked UCB? Supporters argue that there has been enough evidence thus far to start a bank, however, most people seem to agree that too much is unknown about iPSCs and their use in humans. With that, most are in agreement that iPSC research is absolutely needed so banking can become a reality in the future.
For now iPSCs will remain in the testing and research phase, however, based on current research, iPSCs have the potential to enhance Cord Blood that has already been banked, perhaps providing some relief to public banks in the future (Broxmeyer, 2010). Though the potential of iPSCs is endless, more work has to be done before they are placed in humans and considered a viable banking system.
Picture Blog — Making mRNAs by In Vitro Transcription for Transgene Expression and R-iPSCs
R-iPS Cell FAQ 2:
What is the expected yield from the in vitro trancription (IVT) reactions?
Performed as described, you should recover around 40 ug RNA from each 40 uL IVT reaction.
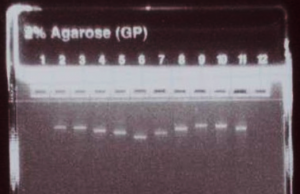
R-iPS Cell FAQ 3:
How can the success of the RNA synthesis protocol be assessed?
Run 500 ng (5 uL) of the concentration-adjusted products on an E-gel to check for consistent product yield and relative product sizes, and to confirm the absence of secondary bands or smears.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008



