differentiation
Picture Blog: A Short Path from Human mRNA-iPSCs to Neurons in Record Speed
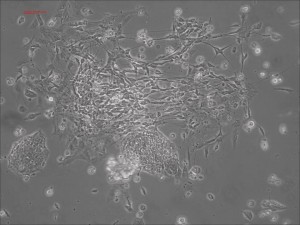
Traditional differentiation protocols use embryoid body (EB) formation as the first step of lineage restriction to mimic early human embryogenesis, which is then followed by manual selection of neuroepithelial precursors. This procedure is tedious and often inconsistent. We have developed a novel neural differentiation scheme that directs human iPSCs (created with the Allele 6F mRNA reprogramming kit) that progressed, as attached culture, to neural precursor cells (NPCs) in just 4-6 days, half the time it typically takes by other methods. From NPCs it takes about another 5-6 days for neural rosettes to form (see figures below); upon passage, cells in neural rosettes differentiate into neurons in 24 hours.
The neural progenitors at the rosettes stage can be stocked and expanded, before differentiated into different types of neurons. We are working on specifically and efficiently different these neural progenitor cells into dopaminergic, glutamatergic, GABAergic, and other types of sub-types of neurons with Allele’s technologies (Questions? email the Allele Stem Cell Group at iPSatAllelebiotech.com).
Neural rosettes formed efficiently in wells without going through EB.
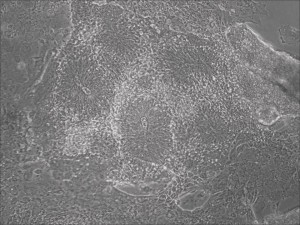
Human iPSC-derived neurons are created in a short regimen developed at Allele Biotech
Monitoring the Undifferentiated Stage of Stem Cells—the Pluripotency Markers
Human embryonic stem (ES) cells or induced pluripotent stem (iPS) cells promise to serve as an unlimited source for transplantation or tissue-specific differentiation. However, obtaining and maintaining stem cells are very difficult tasks for multiple reasons. For instance, most stem cell lines tend to spontaneously differentiate in culture, and even if the cells form stem cell-like colonies, they may be of a heterogeneous population.
To identify pluripotency of stem cells, expression of stem cell-specific marker genes (i.e. Oct-3/4, Sox2, Nanog, Rex-1) is monitored by RT-PCR. Alkaline phosphatase activity and methylation profiles of promoters of pluripotency-relevant genes are often analyzed as well. Compared to murine cells, it is noticeably more difficult to obtain human iPSCs, of which stem cell-like colonies sometimes turn out not to be pluripotent cells. We highly recommend testing iPSCs, especially human iPSCs, with antibodies against stage-specific embryonic antigens such as SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81.
However, all of these methods require cell destruction or fixation for analysis, therefore, are inconvenient and costly. Furthermore, many studies using ES or iPS cells involve differentiation of stem cells into different lineages, a method for observing live cells to know their undifferentiation/differentiation stages would be very helpful. There have been a number of publications using murine Oct-4, Nanog, and Rex-1 promoter driven fluorescent proteins as markers for pluripotency tests [1-3]. Allele Biotech provides, under its iPS product line, packaged and validated lentiviral particles that would insert these 3 promoter-FP reporters into the stem cells. Although currently these promoters are of mouse sequences, their use in human stem cells have been reported.
- New product of the week 01-25-10 to 01-31-10:
All-In-One-Vector: Human OSKM Lentiviral Paticles, with Oct-4, Sox-2, Klf, and c-Myc all expressed from a single virus, ready-to-use.
-
- Promotion of the week:
human iPS cell detection primer set, the same as the landmark Yamanaka paper [4] on creating human iPS for the first time.
1. Da Yong WU, Zhen YAO (2005). Isolation and characterization of the murine Nanog gene promoter. Cell Research, 15 (5): 317–324.
2. Rachel Eiges, Maya Schuldiner..et.al (2001). Establishment of human embryonic stem cell?transfected clones carrying a marker for undifferentiated cell. Current Biology 11: 514–518.
3. Guangjin Pan, Jun Li, Yali Zhou, Hui Zheng, and Duanqing pei (2006). A negative feedback loop of transcription factors that control stem cell pluripotency and self?renewal. ASEB Journal 20: E1094? E1102
4. Takahashi et al, Induction of Pluripotent Stem Cell from Adult Human Fibroblasts by Defined Factors (2007). Cell 131, 861-872
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008



