iPSCs
Picture Blog: Naive Human Pluripotent Stem Cells Regrown From Allele’s iPSCs
As we blogged a month ago, the Hanna lab recently published a paper in Nature describing that human ESCs or iPSCs, which typically resemble more of mouse EpiSCs (epiblast stem cells) than ground state mouse stem cells, could be converted to naïve pluripotent stem cells if grown in a stem cell medium that includes hLIF, JNKi, and p38i. The figure here shows that the reported system did perform well when we at Allele Biotech tested growing our banked iPSCs under similar conditions. The colonies grown in naive stem cell conditions (B) did become dome-shaped when cultured for longer period of time; when transferred back into regular stem cell medium, the once naive-looking iPSCs formed tighter and “cleaner” colonies than typical “primed” human iPSC colonies.
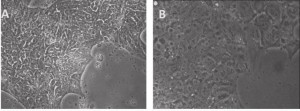
A, Primed human stem cells: mRNA-iPSC line J-1 grown on CellStar-coated surface and in E8 medium. The cells have human iPSC morphology of being compact in size and in "shiny" colonies. B, Naïve human stem cells: J-1 iPSCs shown 2 days after switching to a medium similar to the Naïve Human Stem cell Medium (NHSM). Compared to primed stem cells in A, the naïve stem cells are more flat and transparent, with no spontaneous differentiation on the edges.
New Allele Biotech Publication on Stem Cells
Feeder-Free Reprogramming of Human Fibroblasts with Messenger RNA
Current Protocols in Stem Cell Biology • November 13, 2013
DOI: 10.1002/9780470151808.sc04a06s27
Authors: Luigi Warren, Jiwu Wang
This unit describes a feeder-free protocol for deriving induced pluripotent stem cells (iPSCs) from human fibroblasts by transfection of synthetic mRNA. The reprogramming of somatic cells requires transient expression of a set of transcription factors that collectively activate an endogenous gene regulatory network specifying the pluripotent phenotype. The necessary ectopic factor expression was first effected using retroviruses; however, as viral integration into the genome is problematic for cell therapy applications, the use of footprint-free vectors such as mRNA is increasingly preferred. Strong points of the mRNA approach include high efficiency, rapid kinetics, and obviation of a clean-up phase to purge the vector. Still, the method is relatively laborious and has, up to now, involved the use of feeder cells, which brings drawbacks including poor applicability to clinically oriented iPSC derivation. Using the methods described here, mRNA reprogramming can be performed without feeders at much-reduced labor and material costs relative to established protocols.
Allele iPSC Service and Technology Licensing Contact: http://www.allelebiotech.com/cell-line-and-culture-services/#ips-line
New Allele Product of the Month: FP-nAb™ products for 100% pull-down
Human Pluripotent Stem Cells Are Getting Naïve
In the field of stem cell studies there has been a long standing notion that human embryonic stem cells (ESCs) are not equivalent to mouse ESCs isolated from mouse inner cell mass of blastocyst. When induced pluripotent stem cells (iPSCs) were developed by the Yamanaka lab from human adult cells, they were found to be closer to human ESCs but not as “naïve” as the mouse ESCs.
To learn more, see the following key points about naïve stem cell:
The ground state of human iPSCs or ESCs remains the holy grail in stem cell research largely because of its conceptual value, and also because it was difficult to achieve. When mRNA reprogramming was first described by Warren et al. 2010, the hope was that the mRNA-iPSCs could be closer to ground state compared to virus-mediated iPSCs since mRNA-iPSCs had no issue with uncontrolled transgene expression or silencing. However, the human mRNA- iPSCs produced even with our current, much more potent mRNA mix did not grow in dome-shaped colonies like mouse ESCs, making us wonder whether that is achievable. A recent publication by the Hanna group showed that a ground state pluripotency could be achieved by simply growing cells in the presence of a few additional medium factors, mostly controlling signaling pathways. Since it has been shown that the currently available “primed” (not naïve) human iPSCs can already be derived into various tissue types, the practical impact of the new discovery might be more likely found in removing epigenetic memory after reprogramming, or line-to-line variations if a truly naïve state could be achieved.
Technically, any existing human ESCs or iPSCs could be converted to naïve stem cells, according to the new publication. And when the new medium system, termed NHSM for Naïve Human Stem Cell Medium, was applied to iPSCs, it was used 4 days after the start of the reprogramming run.
Key points about naïve stem cells:
1) Stem cells grow in dome-shaped colonies under 2i/LIF conditions.
2) Doubling time is around 14 h compared to 26 h of primed PSCs.
3) Up to 88% single-cell cloning efficiency in the presence of ROCK inhibitor.
4) OCT4 distal enhancer is used more than the proximal enhancer in naïve PSCs.
5) In cells from female donors, naïve iPSCs are at pre-X inactivation state.
6) More E-CADHERIN expression on the surface of naïve stem cells.
7) It is easier to perform gene targeting by homologous recombination in naïve PSCs.
8) Less H3K27me3 in development genes in naïve cells.
9) High efficiency of integration and chimaerism when naïve iPSCs were injected into mouse embryos.
Gafni et al. “Derivation of novel human ground state naive pluripotent stem cellsNature 2013” http://www.nature.com/nature/journal/vaop/ncurrent/full/nature12745.html
New Product of the Month: Nano antibody Line including GFP-nAb and mNeonGreen-nAb for co-IP using fluorescent proteins as precipitation tag, nab your complexes like never before!
Human iPSC Commercial Service and Technology Licensing: mRNA-iPSCs made feeder-free, xeno-free, and footprint-free.
Picture Blog: How Do You Like Your iPSCs, Clonal or Bulk-Conversion?
Reprogramming of differentiated cells into induced pluripotent stem cells (iPSCs) is commonly considered a stochastic process, i.e. with randomness, which offers an excuse for the commonly seen low efficiency and low constancy of making iPSCs. We have demonstrated time and again that by using potent mRNA cocktails, the majority of the fibroblasts seeded in a well can be converted into pluripotent stage in a nearly synchronized manner (Warren et al. 2012, Warren and Wang 2013, and this Allele Picture Blog series). mRNA molecules can function robustly yet transiently while avoiding the need of entering the nucleus, a bottle-neck for all DNA-based vehicles.
Other researchers are used to the idea of clonal expansion partly because isolating iPSCs from “clones” was a common step during reprogramming using viruses or other low efficiency methods, even though those clones were not necessarily from single precursor cells. This week, the Allele iPSC team developed a new way of managing our mRNA reprogramming that allowed us to achieve clonal iPSCs that appear to be a lot purer and more likely true clones compared to previous reports, without compromising any of the main benefits of our protocol, e.g. feeder-free, xeno-free, footprint-free, very fast and highly efficient. This work is currently supported by an NIDA/NIH grant to Dr. Jiwu Wang at Allele Biotech.
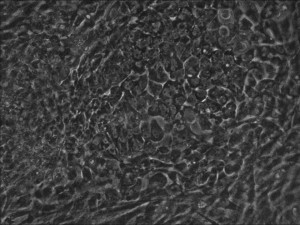
Traditional bulk-conversion by the Allele mRNA reprogramming protocol developed by Warren et al. The picture shows large patches of cells becoming stem cells almost overnight around the 9th day of adding mRNA-cocktail supplement to the media.
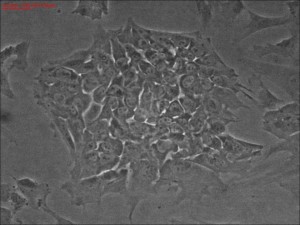
Clonal iPSC formation using a modified mRNA reprogramming protocol. The picture shows a typical clone of stem cells that originated from likely single cells.
Warren, Ni, Wang, and Guo, Scientific Reports, 2012
Warren and Wang, Current Protocols, 2013, in press
Picture Blog: mRNA Reprogramming for Human iPSCs without B18R!
Human induced pluripotent stem cells provide a great route towards personalized medicine and high accuracy drug screening. Allele Biotech has developed the most efficient method of making human iPSCs by using enhanced mRNAs, which have been adopted by leading pharmaceutical companies for clinical trials. The effects from medium-supplementing mRNAs are robust yet transient, and highly specific compared to both miRNAs (off-targets) and small molecules (unknown targets). To repress cellular immune response to introduced RNA molecules, viral protein B18R was previously used during mRNA reprogramming.
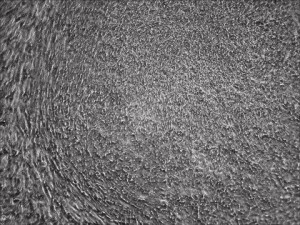
B18R is relatively expensive and inconvenient to use because it requires pre-aliquoting and -80C storage. The protocol has recently been dramatically improved at Allele through an NIDA-funded project. In our latest reprogramming run, all we needed to do was to include mRNA complex in the supplement during medium change for just a week without the need of adding any other type of molecules (such as B18R, miRNA, or chemicals) to help the mRNA mix, unlike all other known mRNA-reprogramming protocols. This advancement can make reprogramming human fibroblasts to footprint-free and xeno-free iPSCs a routine experiment for any lab to perform.
Human R-iPSCs were created without the need of B18R, dramatically reduced the cost and inconvenience. Shown is a newly formed iPSC colony.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008



