pluripotency
Picture Blog: How Do You Like Your iPSCs, Clonal or Bulk-Conversion?
Reprogramming of differentiated cells into induced pluripotent stem cells (iPSCs) is commonly considered a stochastic process, i.e. with randomness, which offers an excuse for the commonly seen low efficiency and low constancy of making iPSCs. We have demonstrated time and again that by using potent mRNA cocktails, the majority of the fibroblasts seeded in a well can be converted into pluripotent stage in a nearly synchronized manner (Warren et al. 2012, Warren and Wang 2013, and this Allele Picture Blog series). mRNA molecules can function robustly yet transiently while avoiding the need of entering the nucleus, a bottle-neck for all DNA-based vehicles.
Other researchers are used to the idea of clonal expansion partly because isolating iPSCs from “clones” was a common step during reprogramming using viruses or other low efficiency methods, even though those clones were not necessarily from single precursor cells. This week, the Allele iPSC team developed a new way of managing our mRNA reprogramming that allowed us to achieve clonal iPSCs that appear to be a lot purer and more likely true clones compared to previous reports, without compromising any of the main benefits of our protocol, e.g. feeder-free, xeno-free, footprint-free, very fast and highly efficient. This work is currently supported by an NIDA/NIH grant to Dr. Jiwu Wang at Allele Biotech.
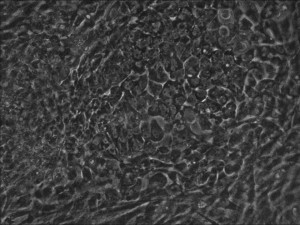
Traditional bulk-conversion by the Allele mRNA reprogramming protocol developed by Warren et al. The picture shows large patches of cells becoming stem cells almost overnight around the 9th day of adding mRNA-cocktail supplement to the media.
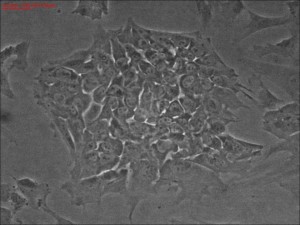
Clonal iPSC formation using a modified mRNA reprogramming protocol. The picture shows a typical clone of stem cells that originated from likely single cells.
Warren, Ni, Wang, and Guo, Scientific Reports, 2012
Warren and Wang, Current Protocols, 2013, in press
Generate mouse and human iPS cells with transfected mature miRNAs
In last week’s blog we discussed generation of induced pluripotent stem cells (iPSCs) with miRNAs expressed from lentivirus. To take it a step further, synthetic, mature miRNAs can be used to avoid the use of viral vectors. Sure enough, Miyoshi et al. published a paper online a few days ago showing that by transfecting 6 miRNAs at 48 hour intervals, they were able to create iPSCs from mouse and human somatic cells. The efficiency is comparable to retrovirus-mediated OSKM factor over-expression (Yoshida et al.), and therefore lower than lentivirus-mediated miR302/369 expression (Anokye-Danso et al.).
In the study of using mature miRNA for obtaining iPSCs, the researchers transfected miRNAs mir200c, mir302s and mir-369 into tissue cultured cells and achieved reprogramming results. Interestingly, only mir302s are common between this study and that with lentivirus-mediated miRNAs by Anokye-Danso et al. There is no current explanation as to why mir-367, which was shown to be required by Anokye-Danso et al., did not seem to be needed in the mature miRNA transfection experiments. Perhaps a level of redundancy among miRNAs, combined with their broad target range and relatively low specificity, allow some of the miRNAs to be interchangeable when used for reprogramming.
Finally, neither of these two recent miRNA-iPSCs works was the first to demonstrate that miRNAs can initiate or facilitate reprogramming. As early as 2008, Lin et al. showed that mir302s could induce pluripotency in a dose-dependent manner by using tet-induced lentivirus expression. They further illustrated that the underlying mechanism is likely through mir302s’ regulation of epigenetic regulators AOFs and other similar factors.
Promotion of the week: Promotion of the week: 10% off on all fluorescent proteins. To redeem, email oligo@allelebiotech.com along with PROMO code: JELLYFISH. See weekly promotions on Facebook.
Telling Good iPSCs from Bad iPSCs
Since its discovery pluripotent stem cells (iPSCs) have been known to differ somewhat from embryonic stem cells (ESCs) in term of gene expression profiles. It also appears that only a small percentage of iPSCs have the full potential of stem cells defined by being able to develop into adult animals. Instead of a global pattern of variations, surprisingly, the difference between iPSC and ESC was found to localize in a small region of one chromosome in mouse, 12qF1, which could account for most iPS cells’ lack of complete pluripotency (Stadtfeld et al, Nature 2010). In this region resides an imprinted gene cluster that includes 2 non-coding genes, Gtl2 and Rian, that remain silenced in most iPSCs. The underlining mechanism is hypermethylation and hypoacetylation, resulting in “paternalizaition” of the region. The effects are manifested around the mid-gestation stage.
By adding histone deacetylase inhibitor valproic acid (VPA) the silenced gene cluster may be reactivated and the iPSCs so treated show increased Gtl2 expression and ability to give rise to normal embryos. Expression of other imprinted genes showed clone-to-clone variations, as was previously reported by a number of groups, but no consistent differences between ESCs cells and iPSCs. Therefore, by analyzing the expression levels of just two genes, Gtl2 and Rian, the potential of iPSCs to be fully pluripotent can be assessed.
The relationships between stem cell status and epigenetic repressions also include the recent finding that Oct4 and Sox2, which are both germ cell-specific and critical reprogramming factors, may be implicated in the regulation of Xist and Tsix RNAs that control epigenetic silencing of X chromosome in female embryos.
New Product of the Week 05-17-10 to 05-23-10: RT-PCR primer set, ABP-SC-iPSh4NX $49, for identifying exogenous iPS factor expression from 4-in-1 iPS lentivirus
Promotion of the Week 05-17-10 to 05-23-10: $85 off IceCube dry bath 0-75C variable temp
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008



