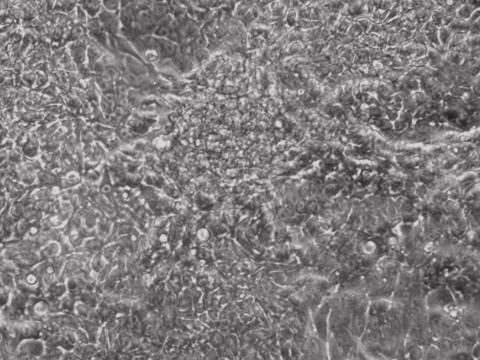
reprogramming
Picture Blog: mRNA Reprogramming for Human iPSCs without B18R!
Human induced pluripotent stem cells provide a great route towards personalized medicine and high accuracy drug screening. Allele Biotech has developed the most efficient method of making human iPSCs by using enhanced mRNAs, which have been adopted by leading pharmaceutical companies for clinical trials. The effects from medium-supplementing mRNAs are robust yet transient, and highly specific compared to both miRNAs (off-targets) and small molecules (unknown targets). To repress cellular immune response to introduced RNA molecules, viral protein B18R was previously used during mRNA reprogramming.
B18R is relatively expensive and inconvenient to use because it requires pre-aliquoting and -80C storage. The protocol has recently been dramatically improved at Allele through an NIDA-funded project. In our latest reprogramming run, all we needed to do was to include mRNA complex in the supplement during medium change for just a week without the need of adding any other type of molecules (such as B18R, miRNA, or chemicals) to help the mRNA mix, unlike all other known mRNA-reprogramming protocols. This advancement can make reprogramming human fibroblasts to footprint-free and xeno-free iPSCs a routine experiment for any lab to perform.
Human R-iPSCs were created without the need of B18R, dramatically reduced the cost and inconvenience. Shown is a newly formed iPSC colony.
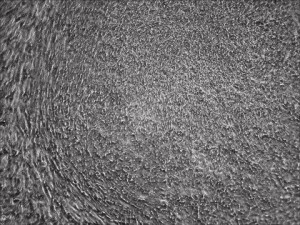
Picture Blog: More Efficient Reprogramming for Creating Induced Stem Cells (iPSCs)
Researchers at Allele Biotech achieved reprogramming of human fibroblasts into iPSCs within one week by including mRNA transfection supplement in daily medium change, at “bulk conversion” efficiency, and with cells seeded at a much wider density range compared to our previous publications. These significant improvements will further facilitate high throughput, large scale iPSC production using Allele’s feeder-free, xeno-free, footprint-free reprogramming, which was already a preferred method for both clinical applications and stem cell banking. The reprogramming project is currently being funded by the NIDA/NIH.
Fusion of the Transcription Domain to iPS Factors Radically Enhances Reprogramming
Induced pluripotent stem cells (iPSCs) can be achieved through introduction of a small group of stem cell specific transcription factors. Ever since this was first demonstrated by Takahashi and Yamanaka, there have been relentless efforts for improving the efficiency of this generally inefficient process. There is also a general opinion that iPSCs are different from each other and from embryonic stem cells (ESCs) in various aspects, depending on the method of the induction. As a result, another focus of the reprogramming field has been to find ways for creating iPSCs that are as close to ESCs as possible. One of the parameters for defining stem cell status is their epigenetic characters; epigenetic changes have been demonstrated to occur during reprogramming of subsequent differentiation.
In fact, it seems that reprogramming can be largely described as a process composed of chromatin remodeling and specific transcription activation. Strong transcription activators are known to effectively recruit multiple chromatin remodeling complexes when exerting their functions. A good example is MyoD, a master transcription factor for skeletal myogenesis that can “single-handedly” switch (transdifferentiate) the fate of differentiated cells. Hirai et al. speculated that since MyoD is such a strong transcription factor, it may be able to increase chromatin accessibility to iPS factors if fused together. When transduced on retroviral vectors, Oct-TAD (Transcription Activation Domain) of MyoD, in combination with Sox2 and Klf4, increased the number of iPSC colonies by 40-fold. Additionally, these iPSCs appeared to quickly adopt stem cell gene expression profiles, days faster than when traditional Oct4, Sox2, c-Myc, and Klf4 were used; and sometimes the levels of pluripotency genes even exceeds those seen in ESCs. Amazingly, when using the fusion assisted method some colonies are formed without the help of feeder cells, a requirement of ESCs grown in similar medium. Does this mean that these iPSCs can even be more “stem-like” than embryonic stem cells?
Like MyoD, VP16, also widely known for its strong transcription activation domain, when fused to iPS factors, was shown to exhibit a similar stimulation effect on reprogramming. Although the details of the fusion arrangements and specificity appear to differ between MyoD and VP16, the fact that two research groups could achieve similar results using comparable strategies provides a good argument that other labs should at least consider this method when creating mouse or human iPSCs. Previously in our blog we have discussed using iPS factor mRNAs, a method originally developed by Warren et al., for substantially shortening the time required for reprogramming and making it more robust across cell types and media conditions. If the new TAD-fusion factors are used also in the mRNA format, then the protocol might be further shortened and simplified. If successful, this non-integrating approach could become a dominant method in the field, even making competitive non-integrating method such as Sendai and plasmid-based miRNA irrelevant.
New product of the week:SurfaceBind RNA purification system, higher capacity and simpler procedure than Qiagen or Ambion’s comparable products, particularly suitable for mRNA cleanup after in vitro transcription.
Promotion of the week: 30% off RNA SurfaceBind Purification kits. To redeem this offer email the code PURIFY to oligo@allelebiotech.com.
Retroviral Vectors with Integrated oriP/EBNA1 for IPSC
The new product of the week of Sep 21-27 is the retrovirus plasmid sets that contain a built-in episomal expression system. As we have discussed previously, OriP/EBNA1 system originated from Epstein-Bar virus, which allows the establishment of stable episomes at 5-20 copies per cell, and duplication once per cell division.
By using the oriP/EBNA1 episomal system, reprogramming cDNAs can be expressed at prolonged time period in reference to plasmid transfection, without integration into chromosomal DNA. A paper published in PLoS One on Sep 18, 2009 by Marchetto et al. showed that by using such a system (on different plasmids) the authors were able to create induced pluripotent stems cells (iPS cells,) effectively from human embryo neural precursor cells.
The Allele pCHAC-EBNA system has dual functions: it can be ready-to-use plasmids for episomal expression of Oct4, Sox2, c-Myc, Klf4, or Nanog and Lin28 by a simple transfection into target cells; it can also be packaged into retroviruses by transfecting into the Allele Phoenix Retrovirus packaging Eco or Ampho cells. This product group is officially launched today. It should become a highly convenient and unique tool for iPSC-related studies.
Launch of Allele-iPS Product Line
Induced pluripotent stem cells, or iPS (or iPSC as some call it), are differentiated cells from adult that “regained” capabilities to differentiate into all 3 germ layer specific cell types. The iPS induction process currently involves using viral vectors to introduce 3 or 4 cDNAs, which seemed surprisingly simple considering how complex it is for stem cells to go through each differentiation pathway.
The potentials of using iPS as models for research, cell assay systems, drug screening, toxicological testing, etc., seem to be tremendous at this point. However, for therapeutic use, the biggest hurdle standing in the way is the tendency of these iPS cells to form tumors once transplanted. It could be due to the oncogenic nature of the stemness inducing cDNAs themselves, or the retrovirus or lentivirus used for bring the cDNAs into the cells. A number of labs like that of Sheng Ding at Scripps, San Diego (Li et al., 2009), and Doug Melton at Harvard (Huangfu et al., 2008a; Huangfu et al., 2008b), are screening chemicals that would replace the use of some or maybe eventually all of the cDNAs. Such advances may help mitigate the oncogenic effects possibly associated with the inducing genes. Using non-integrating vectors as carriers would be preferred method for gene transfer if the retroviral or lentiviral vectors are the cause of tumors from iPS.
Today is the day that Allele launches its iPS product line, officially in this exciting field as one of the very first companies that produce products to make iPS research easier for everybody. New products in the pipeline include those for iPS induction and detection, stem cell culturing, differentiation tracking, and safer, novel delivery methods. It is just the beginning!
Huangfu, D., Maehr, R., Guo, W., Eijkelenboom, A., Snitow, M., Chen, A.E., and Melton, D.A. (2008a). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature biotechnology 26, 795-797.
Huangfu, D., Osafune, K., Maehr, R., Guo, W., Eijkelenboom, A., Chen, S., Muhlestein, W., and Melton, D.A. (2008b). Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature biotechnology 26, 1269-1275.
Li, W., Wei, W., Zhu, S., Zhu, J., Shi, Y., Lin, T., Hao, E., Hayek, A., Deng, H., and Ding, S. (2009). Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell stem cell 4, 16-19.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008