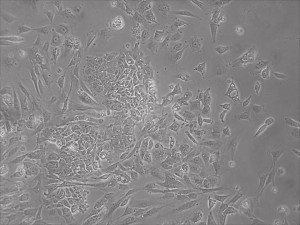
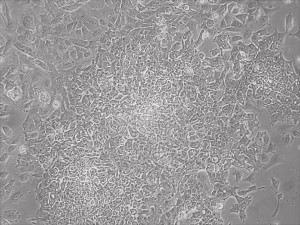
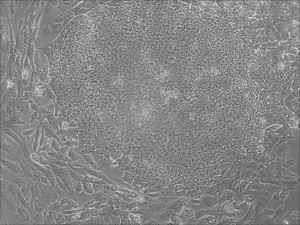
iPSC
Appearance of iPSCs–Different Reprogramming Stages within the Same Well
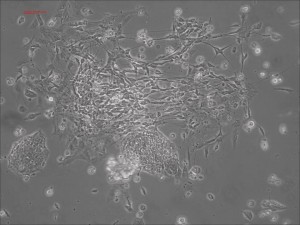
Previously scientists at Allele Biotech have reported near uniform conversion of human fibroblasts using our proprietary mRNA mixtures. The first picture below shows a well of cells after 7 days of growing fibroblasts with the new Allele mRNA mix.
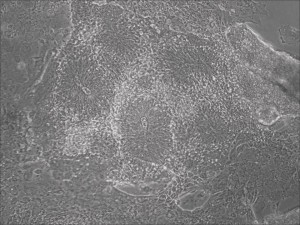
This month, by adjusting the mRNA dose while testing Allele’s own reprogramming medium formulation, we observed various stages of cells going through the transition in the same well (see pictures 2 to 5). All stages of reprogramming typically observed over a span of weeks can actually be seen within 1 well of a 6-well plate when we treated human fibroblasts at half the dose of our standard mRNA mix, on day 10, and using Allele Biotech’s new formulation of reprogramming medium.
(1) Warren, Ni, Wang, and Guo 2012 (pdf download)
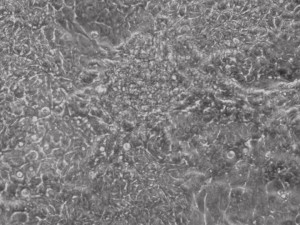
Previous bulk conversion on Day 7 of reprogramming at full dose mRNA, improved upon our published efficiency (1)
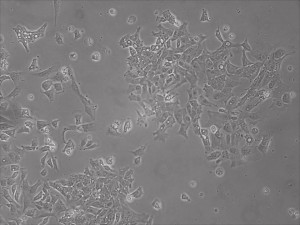
Reprogramming en masse: post mesenchymal-to-epithelial (MET) transition cells start to become iPSCs without surrounding fibroblasts (as opposed to the above figure)
Picture Blog: A Short Path from Human mRNA-iPSCs to Neurons in Record Speed
Traditional differentiation protocols use embryoid body (EB) formation as the first step of lineage restriction to mimic early human embryogenesis, which is then followed by manual selection of neuroepithelial precursors. This procedure is tedious and often inconsistent. We have developed a novel neural differentiation scheme that directs human iPSCs (created with the Allele 6F mRNA reprogramming kit) that progressed, as attached culture, to neural precursor cells (NPCs) in just 4-6 days, half the time it typically takes by other methods. From NPCs it takes about another 5-6 days for neural rosettes to form (see figures below); upon passage, cells in neural rosettes differentiate into neurons in 24 hours.
The neural progenitors at the rosettes stage can be stocked and expanded, before differentiated into different types of neurons. We are working on specifically and efficiently different these neural progenitor cells into dopaminergic, glutamatergic, GABAergic, and other types of sub-types of neurons with Allele’s technologies (Questions? email the Allele Stem Cell Group at iPSatAllelebiotech.com).
Neural rosettes formed efficiently in wells without going through EB.
Human iPSC-derived neurons are created in a short regimen developed at Allele Biotech
New Allele Biotech Publication on Stem Cells
Feeder-Free Reprogramming of Human Fibroblasts with Messenger RNA
Current Protocols in Stem Cell Biology • November 13, 2013
DOI: 10.1002/9780470151808.sc04a06s27
Authors: Luigi Warren, Jiwu Wang
This unit describes a feeder-free protocol for deriving induced pluripotent stem cells (iPSCs) from human fibroblasts by transfection of synthetic mRNA. The reprogramming of somatic cells requires transient expression of a set of transcription factors that collectively activate an endogenous gene regulatory network specifying the pluripotent phenotype. The necessary ectopic factor expression was first effected using retroviruses; however, as viral integration into the genome is problematic for cell therapy applications, the use of footprint-free vectors such as mRNA is increasingly preferred. Strong points of the mRNA approach include high efficiency, rapid kinetics, and obviation of a clean-up phase to purge the vector. Still, the method is relatively laborious and has, up to now, involved the use of feeder cells, which brings drawbacks including poor applicability to clinically oriented iPSC derivation. Using the methods described here, mRNA reprogramming can be performed without feeders at much-reduced labor and material costs relative to established protocols.
Allele iPSC Service and Technology Licensing Contact: http://www.allelebiotech.com/cell-line-and-culture-services/#ips-line
New Allele Product of the Month: FP-nAb™ products for 100% pull-down
mRNA Delivery And the Next Wave of Regenerative Medicine
Published online by Nature Biotechnology, researchers from Ken Chien’s lab at Harvard and other coauthors showed that modified mRNA of VEGF-A injected intramyocardially resulted in the expansion and directed differentiation of endogenous heart progenitors. VEGF-A modRNA markedly improved heart function and enhanced long-term survival of recipients by directing epicardial progenitor cells toward cardiovascular cell types. This publication appears to be the first example of using mRNA as a delivery platform for cell fate-related therapy. AstraZeneca recently invested $240 million on mRNA-related delivery via Moderna, a company with roots within the Harvard stem cell group.
The drastically increased efficacy of using the mRNA platform was accredited to the pulse-like kinetics of mRNA expression profile. It was explained by the fact that native paracrine signals are often transient and precisely regulated in time and space, therefore the pulse-like expression profile of modRNA might be well suited to delivering paracrine-factor signals. Transfected mRNA molecules do not need to penetrate the nuclear membrane, which greatly enhances the efficiency of protein expression on a per transfected molecule over DNA. mRNAs turn over in a much faster pace than plasmid-mediated transgene expression. This is beneficial to many cell fate decisions as exemplified by this recent publication.
Allele Biotech’s reprogramming technologies, licensed by some of the leading stem cell therapy companies, are built around the mRNA platform. We chose mRNA as our core technology to not only change cell fate, but also direct differentiation. We know this platform is the future for cell fate manipulation because we have seen how robustly mRNA expression made the day-and-night difference in gene expression when compared to plasmid DNA (episomal or not), retrovirus, lentivirus, baculo virus, or even transfected proteins. We could convert human fibroblasts into iPSCs, in bulk, in as short as one week with no more effort than changing mRNA complex-containing medium.
Another recent development in iPSC research is in situ reprogramming. Abad et al. generated mice carrying a Tet-inducible cassette of the four cell-reprogramming factors. They then added feed doxycycline to the animals. After several weeks, teratomas appeared in various tissues, indicating that in situ reprogramming had occurred. The iPSCs created this way did not appear to have much advantage over in vitro produced iPSCs other than they are totipotent (helpful if you are studying placenta). Nevertheless, the concept of changing cell fate in situ as dramatically as complete reprogramming is an important leap of faith. As for the next big step, it is easy to see that mRNAs are well suited for in situ reprogramming, as well as transdifferentiation, and more complex gene delivery than the above mentioned VEGF-A alone in heart treatment.
References:
Zangi et al. Nature Biotechnology, http://www.nature.com/nbt/journal/vaop/ncurrent/abs/nbt.2682.html
Abad et al. Nature, http://www.nature.com/nature/journal/vaop/ncurrent/abs/nature12586.html
Allele Biotech Receives $200,000 Grant to Update Its mRNA Reprogramming Commercial Products and Services
On June 10, 2013 Allele received an SBIR award from the National Institute of Drug Abuse (NIDA/NIH) entitled “Revolutionary Technology for Efficient Derivation of Human iPSCs with Messenger RNA”. The goal of the proposed project is to provide to the biomedical research market an advanced reagent kit and services for highly efficient reprogramming of high quality human induced pluripotent stem cells (iPSCs). At the core of this kit is the Allele team’s recent development transcribed messenger RNA (mRNA). Compared to other reprogramming methods, such as lentivirus, Sendai virus, protein, small molecules or any combinations of these reagents, our new generation of the mRNA method often requires less than half the time while sometimes achieving “bulk conversion” efficiency.
While the Allele reprogramming technology was designed for clinical use as the process is feeder-free, xeno-free, chromosome integration-free, as well as without the need for cell splitting, PI, Dr. Jiwu Wang states, “Our purpose of executing the NIH-funded research it to make our method so easy that any researcher can integrate iPSC into his or her projects.” In addition to the extremely high efficiency, mRNA-generated iPSCs should also be more stable because there are no genetic alterations, more uniform among all clones as there is no clonal event, and ultimately suitable for future autologous cell therapy now that creating iPSCs from patient tissue cells should no longer be the rate-limiting steps.
Allele’s business model is to provide cGMP-grade iPSCs to pharmaceutical companies and perform large scale reprogramming by partnering first with university-affiliated hospitals. Great progress has been made in both directions, which has prompted the initiation of a cGMP unit within Allele’s newly acquired building in San Diego.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008