Combining mRNA-Mediated Phenotype Rescue and CRISPR-Created Isogenic Genome
There has been a good volume of publications on using patient-specific iPSCs for disease modeling. Among them, a recent study by Wang et al. published in Nature Medicine is unique because it not only created cardiomyocytes from both Barth Cardiomyopathy patient and wildtype samples for functional analysis, but also combined some of the most exciting new technologies to strengthen the correlation between gene change and disease.
First, functional rescue by mRNA transgene. After mRNA that encodes wild-type cardiolipin aclation enzyme encoding gene tafazzin (TAZ) was transfected into Barth iPSC-CM cells, their defects in mitochondrial functions were corrected. Second, loss of function by genome editing. When CRISPR was used to make genome changes in wildtype cells that mimicked the disease-specific mutation, we recreated the patient’s iPSC-CM phenotype in otherwise wildtype cells. Third, next generation sequencing to confirm genomic changes. And forth, the cardiomyocyte contractibility was assayed on bioengineered chips.
This paper should set an example of how patient iPSCs should be used to create disease models to the fullest extent of usefulness and reliability. We are true believers of the idea that technology development empowers the advancement of science.
“Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies.” Wang, G., McCain, M.L., Yang, L., He, A., Pasqualini, F.S., Agarwal, A., Yuan, H., Jiang, D., Zhang, D., Zangi, L., Geva, J., Roberts, A.E., Ma, Q., Ding, J., Chen, J., Wang, D.Z., Li, K., Wang, J., Wanders, R.J., Kulik, W., Vaz, F.M., Laflamme, M.A., Murry, C.E., Chien, K.R., Kelley, R.I., Church, G.M., Parker, K.K., Pu, W.T. (2014) Nature Medicine, Jun;20(6):616-23. doi: 10.1038/nm.3545. Epub 2014 May 11.
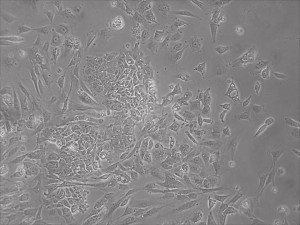
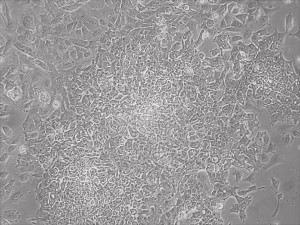
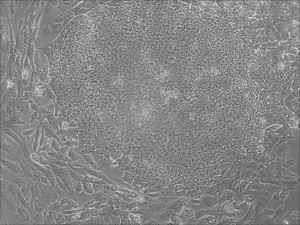
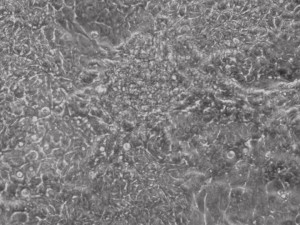
Appearance of iPSCs–Different Reprogramming Stages within the Same Well
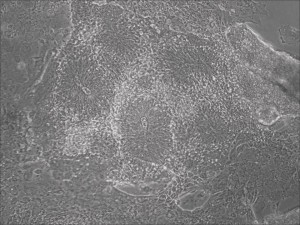
Previously scientists at Allele Biotech have reported near uniform conversion of human fibroblasts using our proprietary mRNA mixtures. The first picture below shows a well of cells after 7 days of growing fibroblasts with the new Allele mRNA mix.
This month, by adjusting the mRNA dose while testing Allele’s own reprogramming medium formulation, we observed various stages of cells going through the transition in the same well (see pictures 2 to 5). All stages of reprogramming typically observed over a span of weeks can actually be seen within 1 well of a 6-well plate when we treated human fibroblasts at half the dose of our standard mRNA mix, on day 10, and using Allele Biotech’s new formulation of reprogramming medium.
(1) Warren, Ni, Wang, and Guo 2012 (pdf download)
Previous bulk conversion on Day 7 of reprogramming at full dose mRNA, improved upon our published efficiency (1)
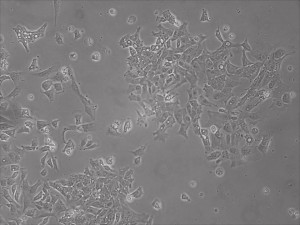
Reprogramming en masse: post mesenchymal-to-epithelial (MET) transition cells start to become iPSCs without surrounding fibroblasts (as opposed to the above figure)
What Does It Take to Bring New Nano Antibodies (nAbs) to the Hands of Researchers?
Judging from the hundreds of papers published using camelid VHH antibodies as reagents, there are probably thousands of researchers who have experience with this type of antibodies by now. We like to call the ~15kD camelid VHH antibody nano antibody or nAbTM. Once someone experiences how well a nAb works for co-IP using a fluorescent protein as tag, they often wonder what it takes to bring nAbs to broader use.
The success of a nAb project starts with the antigen presentation. It is critical to build the capability to produce large quantities of recombinant antigen for immunization. At Allele, our scientists also established some unique presentation formats for traditionally difficult targets (e.g. large membrane proteins).
After llama immunization, the next step is screening. With the goal of creating large scale nano antibodies against diverse targets, we have developed multiple high throughput screening methods to cover very large, diverse libraries generated from immunized animals. The technologies will continue to evolve as the scale of nAb generation continues to expand. We have the ability to functionally screen for site-blocking antibodies and antibodies that only recognized natively folded targets, or targets in their naturally occurring presentations.
A nAb isolation project does not end with the obtaining of a cDNA clone. Or, if it does, the nAb is probably not as great as what Allele Biotech has been offering. In our hands, all nAbs go through an engineering step beginning with the generation of a 3D structural model of the isolated clone. We use structure-guided design to alter the protein, allowing us to improve its properties. This includes increasing affinity, solubility, or altering the protein to improve performance for specific applications. We also like to use known structures of traditional monoclonal antibodies to assist camelid VHH antibody engineering against specific targets.
With a finalized clone in hand, the next step is to establish protocols for commercial production. The Allele team spends a tremendous amount of effort aimed solely at high-yield, low-cost recombinant VHH antibody production in a variety of formats, so that the costs for other scientists to take advantage of these great reagents can be kept as low as possible.
Last but not the least, nAb labeling, including conjugating stable soluble VHH antibody to solid supports for immunoprecipitation or to fluorophores for detection, requires additional expertise and tight operation control. However, our vision is to have a modular system for antibody labeling that will enable the end user to select from a variety of fluorophores and other detection tags, which can be instantaneously and irreversibly coupled via simple mixing.
Note added: we work with commercial (diagnostic and clinical) partners from developing nAbs all the way to the market. We have expert scientists available to customers and licensees for consultation and troubleshooting antibody- and imaging-related questions and problems.
Picture Blog: A Short Path from Human mRNA-iPSCs to Neurons in Record Speed
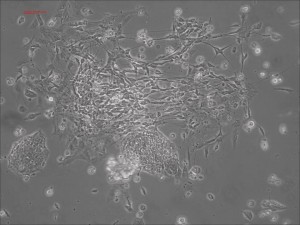
Traditional differentiation protocols use embryoid body (EB) formation as the first step of lineage restriction to mimic early human embryogenesis, which is then followed by manual selection of neuroepithelial precursors. This procedure is tedious and often inconsistent. We have developed a novel neural differentiation scheme that directs human iPSCs (created with the Allele 6F mRNA reprogramming kit) that progressed, as attached culture, to neural precursor cells (NPCs) in just 4-6 days, half the time it typically takes by other methods. From NPCs it takes about another 5-6 days for neural rosettes to form (see figures below); upon passage, cells in neural rosettes differentiate into neurons in 24 hours.
The neural progenitors at the rosettes stage can be stocked and expanded, before differentiated into different types of neurons. We are working on specifically and efficiently different these neural progenitor cells into dopaminergic, glutamatergic, GABAergic, and other types of sub-types of neurons with Allele’s technologies (Questions? email the Allele Stem Cell Group at iPSatAllelebiotech.com).
Neural rosettes formed efficiently in wells without going through EB.
Human iPSC-derived neurons are created in a short regimen developed at Allele Biotech
Allele Biotech Acquires BioCarta’s Distribution Business
Allele Biotechnology & Pharmaceuticals, Inc. is pleased to announce that as of January 1st, 2014, it has acquired the distribution business of BioCarta. This transaction will strengthen Allele Biotech’s presence in the antibody field, enhancing a broad customer and partnership base to further its plan in clinical diagnostics. Biocarta has been a leader in the field of gene expression for 10 years and has contributed immensely through its world leading effort of charting molecular biology pathways. The gene function maps published by Biocarta have been used and referred to by the NIH through the National Center for Biological Information and National Library of Medicine.
Among its well-regarded distribution business, for the past 11 years BioCarta had been the US and Canada’s exclusive distributor of Immune Function Assay Kits for Flow Cytometric Analysis by Glycotope Biotechnology, GmbH. These kits are clinically approved blood cell diagnostic assay products that are also heavily used for non-clinical blood studies.
Allele Biotech has recently launched a major business plan to create a large number of nano antibodies (nAbsTM). The nAbTM line will be great research tools for immunoprecipitation (co-IP), immunohistochemistry (IHC), Western blotting, co-crystallization, biologics purification. Additionally, nAbsTM willbe suitable for diagnostic assays because the single domain antibodies derived from camelid family animals are sturdy, specific, and low-cost. The inclusion of the BioCarta distribution channels in the antibody and pathway reagent fields will help speed up Allele Biotech’s expansion.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008