EpiSCs
Picture Blog: Naive Human Pluripotent Stem Cells Regrown From Allele’s iPSCs
As we blogged a month ago, the Hanna lab recently published a paper in Nature describing that human ESCs or iPSCs, which typically resemble more of mouse EpiSCs (epiblast stem cells) than ground state mouse stem cells, could be converted to naïve pluripotent stem cells if grown in a stem cell medium that includes hLIF, JNKi, and p38i. The figure here shows that the reported system did perform well when we at Allele Biotech tested growing our banked iPSCs under similar conditions. The colonies grown in naive stem cell conditions (B) did become dome-shaped when cultured for longer period of time; when transferred back into regular stem cell medium, the once naive-looking iPSCs formed tighter and “cleaner” colonies than typical “primed” human iPSC colonies.
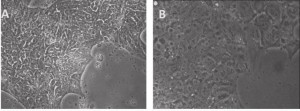
A, Primed human stem cells: mRNA-iPSC line J-1 grown on CellStar-coated surface and in E8 medium. The cells have human iPSC morphology of being compact in size and in "shiny" colonies. B, Naïve human stem cells: J-1 iPSCs shown 2 days after switching to a medium similar to the Naïve Human Stem cell Medium (NHSM). Compared to primed stem cells in A, the naïve stem cells are more flat and transparent, with no spontaneous differentiation on the edges.
Human Pluripotent Stem Cells Are Getting Naïve
In the field of stem cell studies there has been a long standing notion that human embryonic stem cells (ESCs) are not equivalent to mouse ESCs isolated from mouse inner cell mass of blastocyst. When induced pluripotent stem cells (iPSCs) were developed by the Yamanaka lab from human adult cells, they were found to be closer to human ESCs but not as “naïve” as the mouse ESCs.
To learn more, see the following key points about naïve stem cell:
The ground state of human iPSCs or ESCs remains the holy grail in stem cell research largely because of its conceptual value, and also because it was difficult to achieve. When mRNA reprogramming was first described by Warren et al. 2010, the hope was that the mRNA-iPSCs could be closer to ground state compared to virus-mediated iPSCs since mRNA-iPSCs had no issue with uncontrolled transgene expression or silencing. However, the human mRNA- iPSCs produced even with our current, much more potent mRNA mix did not grow in dome-shaped colonies like mouse ESCs, making us wonder whether that is achievable. A recent publication by the Hanna group showed that a ground state pluripotency could be achieved by simply growing cells in the presence of a few additional medium factors, mostly controlling signaling pathways. Since it has been shown that the currently available “primed” (not naïve) human iPSCs can already be derived into various tissue types, the practical impact of the new discovery might be more likely found in removing epigenetic memory after reprogramming, or line-to-line variations if a truly naïve state could be achieved.
Technically, any existing human ESCs or iPSCs could be converted to naïve stem cells, according to the new publication. And when the new medium system, termed NHSM for Naïve Human Stem Cell Medium, was applied to iPSCs, it was used 4 days after the start of the reprogramming run.
Key points about naïve stem cells:
1) Stem cells grow in dome-shaped colonies under 2i/LIF conditions.
2) Doubling time is around 14 h compared to 26 h of primed PSCs.
3) Up to 88% single-cell cloning efficiency in the presence of ROCK inhibitor.
4) OCT4 distal enhancer is used more than the proximal enhancer in naïve PSCs.
5) In cells from female donors, naïve iPSCs are at pre-X inactivation state.
6) More E-CADHERIN expression on the surface of naïve stem cells.
7) It is easier to perform gene targeting by homologous recombination in naïve PSCs.
8) Less H3K27me3 in development genes in naïve cells.
9) High efficiency of integration and chimaerism when naïve iPSCs were injected into mouse embryos.
Gafni et al. “Derivation of novel human ground state naive pluripotent stem cellsNature 2013” http://www.nature.com/nature/journal/vaop/ncurrent/full/nature12745.html
New Product of the Month: Nano antibody Line including GFP-nAb and mNeonGreen-nAb for co-IP using fluorescent proteins as precipitation tag, nab your complexes like never before!
Human iPSC Commercial Service and Technology Licensing: mRNA-iPSCs made feeder-free, xeno-free, and footprint-free.
Creating ground-state human iPSCs
Murine pluripotent stem cells can exist in two distinct states, blastocyst-derived LIF-dependent embryonic stem cells (ESCs) and epiblast-derived bFGF-dependent stem cells (EpiSCs). Murine ESCs and similar iPSC lines are more of the “ground-state” in terms of developmental status, as reflected by the lack of X chromosome inactivation in female cells and their abilities to pass as single cells. Human iPSCs, like human ES cells, are more similar to mouse EpiSCs. Unfortunately these human pluripotent stem cells are difficult to genetically manipulate, e.g. knockin or knockout. They also grow slowly, with doubling time averaging 36 hours. In order to create ground-state human iPSCs, several approaches have been tested, including reprogramming iPSC-derived fibroblasts, continuously expressing 5 iPS factors (Oct4, Sox2, Nanog, c-Myc, and Klf4), or using chemicals to inhibit chromatin modifying enzyme HDAC. While these approaches succeeded to certain degrees, the resulting cell lines seem to have some limitations, such as limited passage numbers.
Retinoic acid (RA) signaling is involved in many aspects of embryonic development. RA receptor (RAR), together with one of its heterodimerization partners, steroid hormone receptor Lrh-1, was recently found to be able to synergize with the 4 common iPS factors (Oct4, Sox2, Klf4, and c-Myc) to induce mouse and human fibroblasts into ground-state iPSCs. The pluripotent cells created by the so-called F6 factor combination show no X chromosome inactivation if from female origin, can fully activate the endogenous Oct4 promoter, express Rex1 (which is specific to mouse ESCs, not EpiSCs), and grow with a 16 hour doubling time. All these mouse ESC-like features were achieved without detectable expression of the exogenous factors once iPSC colonies formed, indicating transient F6 expression is capable of effectively initiating endogenous stem cell factors. Remarkably, these stem cells can maintain their undifferentiated status in mouse ESC medium for 50 passages or more. This work, published this month in Proceedings of National Academy of Science USA [1], provided the stem cell research and application field with a very desirable choice of human stem cells.
As opposed to ~16 days with F4, it appears that the time required to induce adult fibroblasts into pluripotent stem cells is as short as 4 days if F6 factors are introduced on a murine stem cell virus (MSCV) vector with an integrated piggyback transposon. As the authors noted in their discussion, the speed-up benefit should be particularly advantageous for transient transfection approaches such as mRNA reprogramming. The bottom line from this paper and the engineered factor papers (see the previous AlleleBlog article under “iPS and other Stem Cells”) is that iPSC reprogramming is only going to get faster, which means that hopefully in the near future creating iPSCs will become a routine experiment as easy as a simple transfection.
Wang, W., J. Yang, et al. (2011). “Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1.” Proc Natl Acad Sci U S A.
New Products of the week: ARCA, modified cap analog for in vitro transcription of mRNA.
Promotion of the week: Friday special this week, 15% off all iPS viral particle products if using code “ViraliPS” when ordering online at allelebiotech.com, by email, or fax.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008



