ESC
Picture Blog: Naive Human Pluripotent Stem Cells Regrown From Allele’s iPSCs
As we blogged a month ago, the Hanna lab recently published a paper in Nature describing that human ESCs or iPSCs, which typically resemble more of mouse EpiSCs (epiblast stem cells) than ground state mouse stem cells, could be converted to naïve pluripotent stem cells if grown in a stem cell medium that includes hLIF, JNKi, and p38i. The figure here shows that the reported system did perform well when we at Allele Biotech tested growing our banked iPSCs under similar conditions. The colonies grown in naive stem cell conditions (B) did become dome-shaped when cultured for longer period of time; when transferred back into regular stem cell medium, the once naive-looking iPSCs formed tighter and “cleaner” colonies than typical “primed” human iPSC colonies.
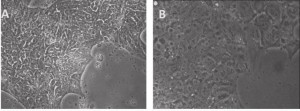
A, Primed human stem cells: mRNA-iPSC line J-1 grown on CellStar-coated surface and in E8 medium. The cells have human iPSC morphology of being compact in size and in "shiny" colonies. B, Naïve human stem cells: J-1 iPSCs shown 2 days after switching to a medium similar to the Naïve Human Stem cell Medium (NHSM). Compared to primed stem cells in A, the naïve stem cells are more flat and transparent, with no spontaneous differentiation on the edges.
Human Pluripotent Stem Cells Are Getting Naïve
In the field of stem cell studies there has been a long standing notion that human embryonic stem cells (ESCs) are not equivalent to mouse ESCs isolated from mouse inner cell mass of blastocyst. When induced pluripotent stem cells (iPSCs) were developed by the Yamanaka lab from human adult cells, they were found to be closer to human ESCs but not as “naïve” as the mouse ESCs.
To learn more, see the following key points about naïve stem cell:
The ground state of human iPSCs or ESCs remains the holy grail in stem cell research largely because of its conceptual value, and also because it was difficult to achieve. When mRNA reprogramming was first described by Warren et al. 2010, the hope was that the mRNA-iPSCs could be closer to ground state compared to virus-mediated iPSCs since mRNA-iPSCs had no issue with uncontrolled transgene expression or silencing. However, the human mRNA- iPSCs produced even with our current, much more potent mRNA mix did not grow in dome-shaped colonies like mouse ESCs, making us wonder whether that is achievable. A recent publication by the Hanna group showed that a ground state pluripotency could be achieved by simply growing cells in the presence of a few additional medium factors, mostly controlling signaling pathways. Since it has been shown that the currently available “primed” (not naïve) human iPSCs can already be derived into various tissue types, the practical impact of the new discovery might be more likely found in removing epigenetic memory after reprogramming, or line-to-line variations if a truly naïve state could be achieved.
Technically, any existing human ESCs or iPSCs could be converted to naïve stem cells, according to the new publication. And when the new medium system, termed NHSM for Naïve Human Stem Cell Medium, was applied to iPSCs, it was used 4 days after the start of the reprogramming run.
Key points about naïve stem cells:
1) Stem cells grow in dome-shaped colonies under 2i/LIF conditions.
2) Doubling time is around 14 h compared to 26 h of primed PSCs.
3) Up to 88% single-cell cloning efficiency in the presence of ROCK inhibitor.
4) OCT4 distal enhancer is used more than the proximal enhancer in naïve PSCs.
5) In cells from female donors, naïve iPSCs are at pre-X inactivation state.
6) More E-CADHERIN expression on the surface of naïve stem cells.
7) It is easier to perform gene targeting by homologous recombination in naïve PSCs.
8) Less H3K27me3 in development genes in naïve cells.
9) High efficiency of integration and chimaerism when naïve iPSCs were injected into mouse embryos.
Gafni et al. “Derivation of novel human ground state naive pluripotent stem cellsNature 2013” http://www.nature.com/nature/journal/vaop/ncurrent/full/nature12745.html
New Product of the Month: Nano antibody Line including GFP-nAb and mNeonGreen-nAb for co-IP using fluorescent proteins as precipitation tag, nab your complexes like never before!
Human iPSC Commercial Service and Technology Licensing: mRNA-iPSCs made feeder-free, xeno-free, and footprint-free.
Telling Good iPSCs from Bad iPSCs
Since its discovery pluripotent stem cells (iPSCs) have been known to differ somewhat from embryonic stem cells (ESCs) in term of gene expression profiles. It also appears that only a small percentage of iPSCs have the full potential of stem cells defined by being able to develop into adult animals. Instead of a global pattern of variations, surprisingly, the difference between iPSC and ESC was found to localize in a small region of one chromosome in mouse, 12qF1, which could account for most iPS cells’ lack of complete pluripotency (Stadtfeld et al, Nature 2010). In this region resides an imprinted gene cluster that includes 2 non-coding genes, Gtl2 and Rian, that remain silenced in most iPSCs. The underlining mechanism is hypermethylation and hypoacetylation, resulting in “paternalizaition” of the region. The effects are manifested around the mid-gestation stage.
By adding histone deacetylase inhibitor valproic acid (VPA) the silenced gene cluster may be reactivated and the iPSCs so treated show increased Gtl2 expression and ability to give rise to normal embryos. Expression of other imprinted genes showed clone-to-clone variations, as was previously reported by a number of groups, but no consistent differences between ESCs cells and iPSCs. Therefore, by analyzing the expression levels of just two genes, Gtl2 and Rian, the potential of iPSCs to be fully pluripotent can be assessed.
The relationships between stem cell status and epigenetic repressions also include the recent finding that Oct4 and Sox2, which are both germ cell-specific and critical reprogramming factors, may be implicated in the regulation of Xist and Tsix RNAs that control epigenetic silencing of X chromosome in female embryos.
New Product of the Week 05-17-10 to 05-23-10: RT-PCR primer set, ABP-SC-iPSh4NX $49, for identifying exogenous iPS factor expression from 4-in-1 iPS lentivirus
Promotion of the Week 05-17-10 to 05-23-10: $85 off IceCube dry bath 0-75C variable temp
FAQ About Feeder Cells for Stem Cells –Part One
The cost of preparing feeder cells for induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) is mainly due to 1. serum and media, 2. labor for growing and treating cells, and 3. expenses for freezing media and vials. Ready-to-use feeder cells saves one important labor-intensive step of iPSC generation, it should be an important help for iPSC and stem cell researchers. We know that most of our colleagues are tired of preparing fresh early passages of MEFs and treating them with expensive mitomycin C or finding an irradiator to pre-treat the MEFs. A lot of iPSC researchers lost iPS stem cells due to the lack of patience in handling MEF feeders. The offering of Allele’s feeder cell product line is really an easy solution and convenience to iPSC researchers.
Question 1: There are companies offering drug-resistant feeder cells such as MEF cells expressing neo-, puro-, or hygromycin-resistance genes. Is it important to have such drug-resistance genes when choosing feeder cells?
Adding drug resistant markers to these cells should not be necessary because iPSCs grown on feeder cells are usually not cultured in antibiotics-containing medium. The feeder cells will not be selected by drug resistance nor will they contaminate iPS cells since they can not propagate after irradiation. However, for those who do need to use drug selection for any reason, we will provide drug-resistant feeder cells upon request.
Question 2: There are publications showing the use of cells lines as feeder cells instead of primary fibroblasts, e.g. SL10, MRC-5, STO. Are there any advantages of using these cell lines?
Not really. Handling primary cells requires certain amount of experience and may be tedious; using cell lines, on the other hand, would be easier for preparing feeder cells. We provide feeder cells from immortalized early passage human foreskin fibroblasts at prices often lower than those from cell lines.
Question 3: Should I choose fluorescent protein expressing feeder cells for easy separation from iPSCs?
You do not need to include fluorescent protein in feeder cells, as feeder cells are quite different in morphology from iPS cells or ES cells. In fact, many labs use iPS factors that are co-expressed with fluorescent markers, in which cases feeder cell expressed fluorescent proteins will confuse the readout.
Question 4: What are the main advantages of using bFGF-expressing feeder cells?
Our bFGF-feeder cells not only eliminate the needs for added recombinant bFGF to stem cell cultures, but also form very nice cell lawn to serve iPSC colony formation because of their strictly controlled passage and growth conditions. We have used these cells without coating dishes with gelatin and obtained nice iPSC colonies.
Preview: Next Part of FAQ on Feeder Cells: choosing mouse or human fibroblasts, selecting iPSC colonies…
Announcement: An audience-orientated User Forum will be added to Allele Biotech webpages so that people can freely discuss or review products and technologies. A distilled version of discussions will be presented in a related but separate FAQ section, which will also include all Allele eNewsletters sent to our contacts about every quarter. Look for the links on www.allelebiotech.com in coming weeks.
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008



