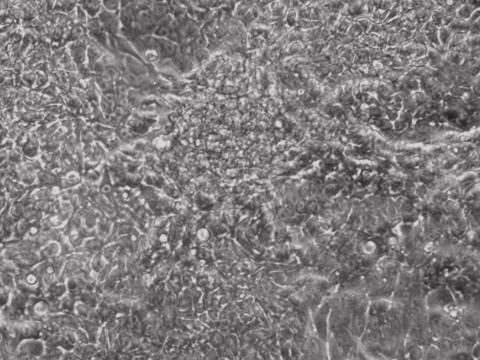
mRNA iPSCs
Picture Blog: Naive Human Pluripotent Stem Cells Regrown From Allele’s iPSCs
As we blogged a month ago, the Hanna lab recently published a paper in Nature describing that human ESCs or iPSCs, which typically resemble more of mouse EpiSCs (epiblast stem cells) than ground state mouse stem cells, could be converted to naïve pluripotent stem cells if grown in a stem cell medium that includes hLIF, JNKi, and p38i. The figure here shows that the reported system did perform well when we at Allele Biotech tested growing our banked iPSCs under similar conditions. The colonies grown in naive stem cell conditions (B) did become dome-shaped when cultured for longer period of time; when transferred back into regular stem cell medium, the once naive-looking iPSCs formed tighter and “cleaner” colonies than typical “primed” human iPSC colonies.
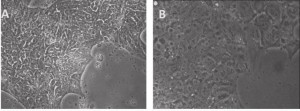
A, Primed human stem cells: mRNA-iPSC line J-1 grown on CellStar-coated surface and in E8 medium. The cells have human iPSC morphology of being compact in size and in "shiny" colonies. B, Naïve human stem cells: J-1 iPSCs shown 2 days after switching to a medium similar to the Naïve Human Stem cell Medium (NHSM). Compared to primed stem cells in A, the naïve stem cells are more flat and transparent, with no spontaneous differentiation on the edges.
Human Pluripotent Stem Cells Are Getting Naïve
In the field of stem cell studies there has been a long standing notion that human embryonic stem cells (ESCs) are not equivalent to mouse ESCs isolated from mouse inner cell mass of blastocyst. When induced pluripotent stem cells (iPSCs) were developed by the Yamanaka lab from human adult cells, they were found to be closer to human ESCs but not as “naïve” as the mouse ESCs.
To learn more, see the following key points about naïve stem cell:
The ground state of human iPSCs or ESCs remains the holy grail in stem cell research largely because of its conceptual value, and also because it was difficult to achieve. When mRNA reprogramming was first described by Warren et al. 2010, the hope was that the mRNA-iPSCs could be closer to ground state compared to virus-mediated iPSCs since mRNA-iPSCs had no issue with uncontrolled transgene expression or silencing. However, the human mRNA- iPSCs produced even with our current, much more potent mRNA mix did not grow in dome-shaped colonies like mouse ESCs, making us wonder whether that is achievable. A recent publication by the Hanna group showed that a ground state pluripotency could be achieved by simply growing cells in the presence of a few additional medium factors, mostly controlling signaling pathways. Since it has been shown that the currently available “primed” (not naïve) human iPSCs can already be derived into various tissue types, the practical impact of the new discovery might be more likely found in removing epigenetic memory after reprogramming, or line-to-line variations if a truly naïve state could be achieved.
Technically, any existing human ESCs or iPSCs could be converted to naïve stem cells, according to the new publication. And when the new medium system, termed NHSM for Naïve Human Stem Cell Medium, was applied to iPSCs, it was used 4 days after the start of the reprogramming run.
Key points about naïve stem cells:
1) Stem cells grow in dome-shaped colonies under 2i/LIF conditions.
2) Doubling time is around 14 h compared to 26 h of primed PSCs.
3) Up to 88% single-cell cloning efficiency in the presence of ROCK inhibitor.
4) OCT4 distal enhancer is used more than the proximal enhancer in naïve PSCs.
5) In cells from female donors, naïve iPSCs are at pre-X inactivation state.
6) More E-CADHERIN expression on the surface of naïve stem cells.
7) It is easier to perform gene targeting by homologous recombination in naïve PSCs.
8) Less H3K27me3 in development genes in naïve cells.
9) High efficiency of integration and chimaerism when naïve iPSCs were injected into mouse embryos.
Gafni et al. “Derivation of novel human ground state naive pluripotent stem cellsNature 2013” http://www.nature.com/nature/journal/vaop/ncurrent/full/nature12745.html
New Product of the Month: Nano antibody Line including GFP-nAb and mNeonGreen-nAb for co-IP using fluorescent proteins as precipitation tag, nab your complexes like never before!
Human iPSC Commercial Service and Technology Licensing: mRNA-iPSCs made feeder-free, xeno-free, and footprint-free.
Picture Blog: More Efficient Reprogramming for Creating Induced Stem Cells (iPSCs)
Researchers at Allele Biotech achieved reprogramming of human fibroblasts into iPSCs within one week by including mRNA transfection supplement in daily medium change, at “bulk conversion” efficiency, and with cells seeded at a much wider density range compared to our previous publications. These significant improvements will further facilitate high throughput, large scale iPSC production using Allele’s feeder-free, xeno-free, footprint-free reprogramming, which was already a preferred method for both clinical applications and stem cell banking. The reprogramming project is currently being funded by the NIDA/NIH.
Fusion of the Transcription Domain to iPS Factors Radically Enhances Reprogramming
Induced pluripotent stem cells (iPSCs) can be achieved through introduction of a small group of stem cell specific transcription factors. Ever since this was first demonstrated by Takahashi and Yamanaka, there have been relentless efforts for improving the efficiency of this generally inefficient process. There is also a general opinion that iPSCs are different from each other and from embryonic stem cells (ESCs) in various aspects, depending on the method of the induction. As a result, another focus of the reprogramming field has been to find ways for creating iPSCs that are as close to ESCs as possible. One of the parameters for defining stem cell status is their epigenetic characters; epigenetic changes have been demonstrated to occur during reprogramming of subsequent differentiation.
In fact, it seems that reprogramming can be largely described as a process composed of chromatin remodeling and specific transcription activation. Strong transcription activators are known to effectively recruit multiple chromatin remodeling complexes when exerting their functions. A good example is MyoD, a master transcription factor for skeletal myogenesis that can “single-handedly” switch (transdifferentiate) the fate of differentiated cells. Hirai et al. speculated that since MyoD is such a strong transcription factor, it may be able to increase chromatin accessibility to iPS factors if fused together. When transduced on retroviral vectors, Oct-TAD (Transcription Activation Domain) of MyoD, in combination with Sox2 and Klf4, increased the number of iPSC colonies by 40-fold. Additionally, these iPSCs appeared to quickly adopt stem cell gene expression profiles, days faster than when traditional Oct4, Sox2, c-Myc, and Klf4 were used; and sometimes the levels of pluripotency genes even exceeds those seen in ESCs. Amazingly, when using the fusion assisted method some colonies are formed without the help of feeder cells, a requirement of ESCs grown in similar medium. Does this mean that these iPSCs can even be more “stem-like” than embryonic stem cells?
Like MyoD, VP16, also widely known for its strong transcription activation domain, when fused to iPS factors, was shown to exhibit a similar stimulation effect on reprogramming. Although the details of the fusion arrangements and specificity appear to differ between MyoD and VP16, the fact that two research groups could achieve similar results using comparable strategies provides a good argument that other labs should at least consider this method when creating mouse or human iPSCs. Previously in our blog we have discussed using iPS factor mRNAs, a method originally developed by Warren et al., for substantially shortening the time required for reprogramming and making it more robust across cell types and media conditions. If the new TAD-fusion factors are used also in the mRNA format, then the protocol might be further shortened and simplified. If successful, this non-integrating approach could become a dominant method in the field, even making competitive non-integrating method such as Sendai and plasmid-based miRNA irrelevant.
New product of the week:SurfaceBind RNA purification system, higher capacity and simpler procedure than Qiagen or Ambion’s comparable products, particularly suitable for mRNA cleanup after in vitro transcription.
Promotion of the week: 30% off RNA SurfaceBind Purification kits. To redeem this offer email the code PURIFY to oligo@allelebiotech.com.
Expression of iPS Factors from Transfected mRNA
Differentiated cells can be reprogrammed to pluripotency by enforced expression of certain combinations of stem cell-specific protein factors in them. The power of this method was first demonstrated by Yamanaka’s group using retroviruses carrying Oct3/4, Sox2, c-Myc, and Klf4. Alternative factors such as Lin28 and Nanog, and additional factors such as the human telomerase gene hTert and shRNA against p53 were also shown to contribute to reprogramming. From the very beginning it was realized that viral integration would pose a major problem in using the induced pluripotent stem cells (iPSCs) for clinical purposes. There have been multiple attempts to circumvent this problem by using non-integrating vectors such as plasmid, minicircle DNA, adenovirus, baculovirus, removable transposons, episomal DNA, or by introducing recombinant proteins with a transmembrane domain into target cells. From reports in the field and customer feedbacks it seems that retroviral or lentiviral systems are still the most efficient in reprogramming. mRNA is about the only option left unreported, until an article by Warren et al was published in Cell Stem Cell online recently.
From that report, it is clear that the reason that it took so long for RNA-induced iPSCs (RiPSCs) to appear in the literature was because synthetic mRNAs activate interferon responses in mammalian cells, reminding us of the early days of RNAi. The authors took a number of steps to reduce interferon responses, including adding a 5’-cap (actually a fairly standard step in in vitro transcription), using a phosphatase to remove 5’ triphosphates on uncapped mRNAs, and using modified C and U bases (5-methucytidine or 5mC and pseudouridine or psi) during T7 promoter-driven in vitro transcription. The prepared mRNA was then administered everyday for 17 days at an amount not clearly defined in the paper. The main benefit of this method is of course that there is no gene integration to alter the chromosome. The efficiency of the new method was also compared to using viral vectors and it was shown that 1.4% conversion efficiency was achieved vs retroviral systems’ 0.01% (although we have experienced better results using lentivirus, at least the 4-in-1 version).
The DNA templates used for in vitro transcription of the iPS factors were created by multiple PCR reactions and bridged ligation; it could also be done by other cloning strategies. For those excited about trying this new way of making iPSCs, the major hassle would be preparing modified mRNAs good and abundant enough for 17 consecutive transfections. Allele Biotech would like to provide custom services, before offering shelf products, for creating such mRNAs as the method sounds potentially very helpful to many researchers in the iPSC field.
- New Product of the Week 100410-101010:
pLICO-mWasabi (Promoterless FP Reporter Vector ), listed as product-on-demand, now available, ABP-HL-PE40010 $395.00.
- Promotion of the Week 100410-101010:
Barrier too high to start using virus? Allele lowers it for starters, $500 for bactulo virus protein production, and $300 retrovirus packaging. Code 100310VIVEC, email vivec@allelebiotech.com
Categories
- Allele Mail Bag
- cGMP
- Customer Feedback
- Fluorescent proteins
- iPSCs and other stem cells
- nAb: Camelid Antibodies, Nanobodies, VHH
- Next Generation Sequencing (NextGen Seq)
- NIH Budget and You
- oligos and cloning
- Open Forum
- RNAi patent landscape
- SBIR and Business issues
- State of Research
- Synthetic biology
- Uncategorized
- Viruses and cells
- You have the power
Archives
- October 2018
- April 2018
- March 2018
- January 2018
- October 2017
- September 2017
- August 2017
- March 2017
- February 2017
- January 2017
- November 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- February 2016
- October 2015
- September 2015
- August 2015
- June 2015
- March 2015
- January 2015
- December 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- May 2012
- April 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
- March 2011
- February 2011
- January 2011
- December 2010
- November 2010
- October 2010
- September 2010
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- December 2009
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- October 2008
- August 2008
- July 2008